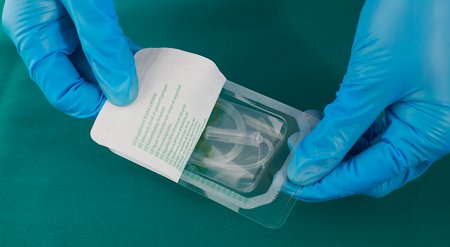
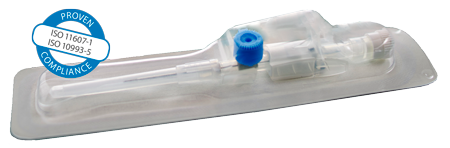
Medical Device Packaging Films
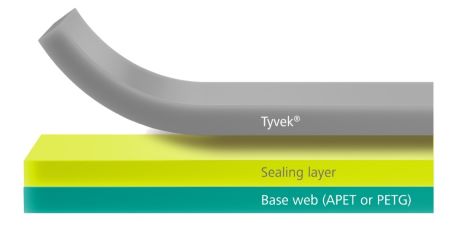
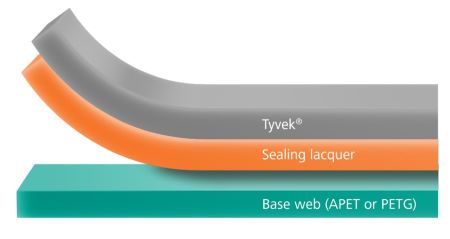
kpSealable® films offer significant cost savings by being designed for use with uncoated, non-woven HDPE lidding films. This innovation eliminates the need for expensive, lacquer-coated lid stocks, making your packaging process more economical. By reducing material costs, kpSealable® helps streamline your operations and improve your bottom line.
Additionally, kpSealable® films are fully compliant with applicable FDA and EU regulations for direct food contact, ensuring that your products meet the highest safety standards. They also adhere to the relevant ISO 11607-1 and ISO 10993-5 regulations, which cover packaging for terminally sterilized medical devices and biocompatibility. This compliance guarantees that kpSealable® not only provides cost-effective solutions but also meets stringent regulatory requirements.
|
|
Benefits
Uncoated lidding improves permeability
Processes on existing equipment
Helps reduce the risk of particle contamination
Cost effective solution compared to a standard film structure
Applications include:
- Wound care kits
- Syringes
- Surgical kits
- Catheters
Need of help with a particular packaging problem? Maybe you are facing issues such as:
- Forming issues
- Migration concerns
- Regulatory/compliance issues
- Design roadblocks
If so, we provide a unique service called BlisterPro® XCEL where we use our packaging, materials, and design expertise to quickly guide you through to a viable solution.
| BlisterPro® XCEL Services |
|
We invite you to explore the comprehensive selection of Pentamed® medical device packaging films. Our diverse product lineup is designed to meet the highest standards of safety, performance, and compliance for medical device packaging.
From advanced barrier films to innovative sustainable options, Pentamed® offers solutions tailored to your specific needs. Each product is engineered to ensure optimal protection, regulatory compliance, and enhanced performance. Discover how Pentamed® can enhance your packaging strategy and contribute to your success!
| Take Me There |
Stay Up-To-Date with kp!
Tyvek® is a registered trademark of Dupont®